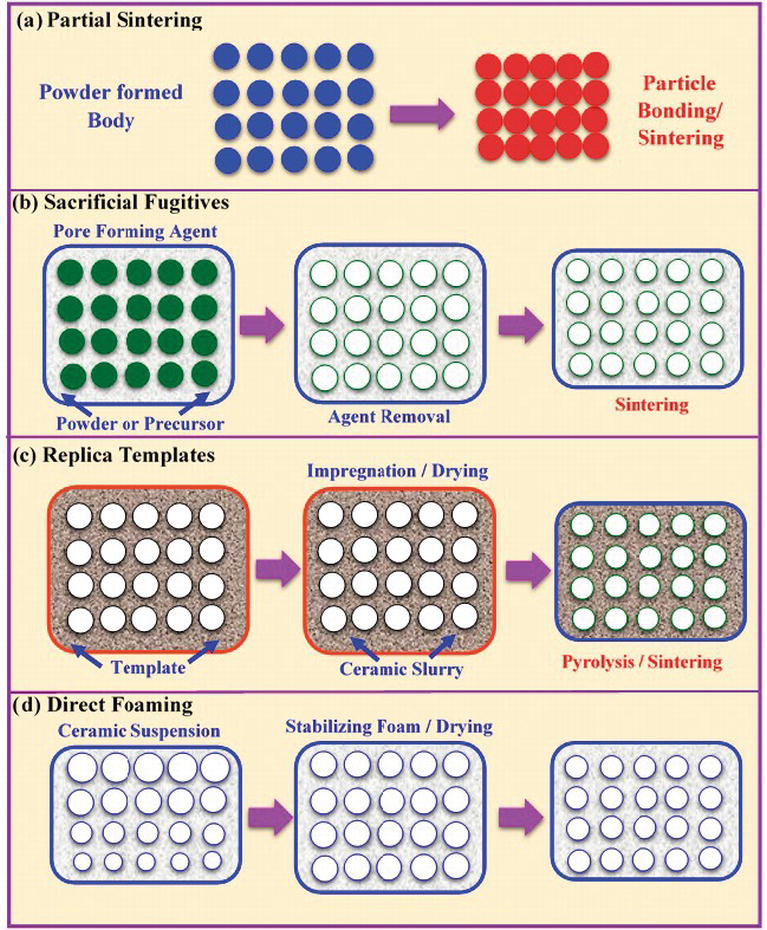
The method involves ion exchange of an appropriate zeolite powder followed by fabrication and sintering to form a dense ceramic.
Use of zeolites in preparing low temperature ceramics.
They are well known in cavities of basalt having crystallized as a result of diagenetic or hydrothermal alteration figure 1 some zeolites completely replace rhyolitic tuff in saline alkaline lacustrine environments or through groundwater.
They can withstand elevated temperatures heavy loads will remain free flowing immersed in water and will not disintegrate when exposed to air.
Zeolites as an adsorbent.
These technologies have some both advantages and disadvantages.
The zeolite facies is generally considered to be transitional between diagenetic processes which turn sediments into sedimentary rocks and prehnite pumpellyite facies which is a hallmark of subseafloor alteration of the oceanic crust around mid ocean ridge.
Zeolites are aluminosilicate minerals that occur as low temperature generally less than 200 c alteration products of volcanic and feldspathic rocks.
The conventional process is used for treatment of h 2 s involving adsorption scrubbing and biological treatment at low temperatures 298 373 k.
At the cement clay interface zeolites can play a major role in the porosity clogging because of the high molar volume of those hydrated phases.
It should also be noted that heating zeolites to very high temperatures can cause extensive structural damage.
There would need to be a chemical analysis of the particular zeolite s in use to determine the characteristics before it could be determined what level of heat is required to sufficiently remove lower levels of moisture.
A novel synthetic route for fabricating dense aluminosilicate based ceramics at relatively low temperatures 1000 c is described.
Addition to c s h phases.
Zeolite facies describes the mineral assemblage resulting from the pressure and temperature conditions of low grade metamorphism.
The xrd patterns shows comparable values to reported ones for commercial zeolites and sem images revealed that the 13x zeolite was pure and exhibited octahedral.
Zeolite lta also known as zeolite a na 12 al 12 si 12 o 48 27h 2 o has a three dimensional pore structure with pores running perpendicular to each other in the x y and z planes and it is made up of secondary building units 4 6 8 and 4 4 as an arrangement of β cage 4 8 6 6 pseudo cornershare via 4 4 structure units showing α.
Zeolites are inert and extremely durable.
The 13x zeolite was synthesized from low grade natural kaolin via alkali fusion followed by hydrothermal treatment 50 c 2 h for homogenization 90 c 8 h for crystallization.
The actual nature of zeolite and c s h were shown to depend especially on kinetic factors time temperature and on the nature of the alkaline solution.